Abstract
Introduction: Randomized controlled trials (RCTs) testing carfilzomib-based combination regimens in myeloma have demonstrated a signal of increased renal toxicity, with a relative risk of 2.3 for grade≥3 toxicities with carfilzomib-based versus non-carfilzomib-based regimens (Ball et al. Annals of Hematology. 2020). Nonetheless, there are insufficient data on detailed characterization of carfilzomib-induced renal toxicity, especially in patients treated with carfilzomib alone or with dexamethasone, without the confounding effect of additional classes of anti-myeloma therapy. The objective of our study was to estimate the incidence, timing, nature, and potential risk-factors of carfilzomib-induced renal toxicity using data from S1304 which compared two doses of carfilzomib (56 mg/m2 (K56) vs 27 mg/m2 (K27) twice-weekly), both with dexamethasone, in relapsed/refractory myeloma (Ailawadhi et al. Clinical Cancer Research. 2020).
Methods: Individual patient-level data from S1304 trial (NCT01903811) were utilized to estimate the incidence, nature, and timing of renal toxicities. The toxicity of interest was acute kidney injury (AKI), in which, we included the adverse event description of "increased creatinine". Baseline characteristics in patients who developed AKI versus those who didn't were compared using Fisher's exact test.
Results: Our analysis on renal toxicity included 123 patients, with 57 patients randomized to K56 and 66 to K27 arms. All-grade AKI/increased creatinine (hereafter, referred to as AKI) was seen in 21 patients overall (17.1%), with grade≥3 toxicity seen in 6 patients (4.9%). By treatment arms, the incidence of all-grade and grade≥3 AKI for K56 was 21.1% and 5.3% respectively, whereas for K27 arm it was 13.6% and 4.5%, respectively. The median time to onset of AKI was 2.5 (range, 2-3) and 2 (range, 1-7) cycles for all-grade and grade≥3 respectively. Kidney toxicities of interest other than AKI were CKD and proteinuria. The overall incidence of all-grade CKD and proteinuria were low at 1.6% and 2.4% respectively, with no grade ≥3 events. The incidence of CKD in K56 and K27 arms was 1.8% and 1.5% respectively, whereas that for proteinuria was 1.8% and 3% respectively. The median time to onset of CKD was 3.5 cycles (range, 3-4) and that of proteinuria was 2 cycles (range, 1-9).
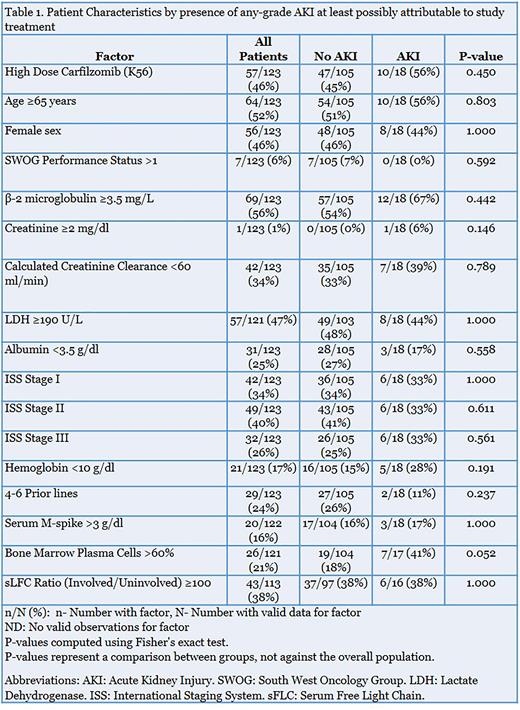
Subsequently, we investigated any potential differences in baseline patient-specific, disease-specific, or treatment-specific characteristics between patients who developed AKI versus those who did not (Table 1). The only variable that showed a near-significant difference between the two was percent bone marrow plasma cells (BMPC), with %BMPC in the two groups being 41% vs 18% respectively (p=0.052). There was no significant difference in the proportion of patients receiving K56 between the two groups (56% vs 45% respectively; p=0.45). Next, we performed a 3-month landmark analysis to estimate progression-free survival (PFS) stratified by development of any-grade AKI that was at least possibly attributable to study treatment prior to 3 months following randomization. There was no significant difference in median PFS between patients who developed kidney toxicity versus those who did not.
Conclusion: In summary, we showed that ≥grade 3 AKI is observed in ~5% of patients receiving carfilzomib plus dexamethasone, with most events occurring early at a median of about 2 cycles from treatment initiation. Furthermore, high tumor burden at baseline is a potential predictor of AKI with carfilzomib, whereas dose-intensity of carfilzomib was not different between patients who developed AKI versus those who didn't. Our findings are comparable to that of the ARROW trial which tested once weekly (70 mg/m2) versus twice-weekly (27 mg/m2) carfilzomib, and demonstrated grade≥3 AKI in 4% and 6% of patients, respectively. Future studies should investigate additional risk-factors and reversibility of carfilzomib-induced renal toxicity as well as impact on survival in larger patient cohorts.
Disclosures
Chakraborty:Adaptive Biotech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Lentzsch:Magenta: Other: Equity Ownership; Pfizer: Consultancy; Poseida: Other: Equity ownership; Takeda: Consultancy; Nataea: Consultancy; Janssen: Consultancy; GlaxoSmithKline: Consultancy; Sanofi: Consultancy, Research Funding; Oncopeptides: Consultancy; Zentalis: Research Funding; Caelum Bioscience: Consultancy, Other: Stock ownership; Peerview: Speakers Bureau; Clinical Care Options: Speakers Bureau; Prothena: Honoraria. Cohen:Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Ichnos, Janssen Oncology, Oncopeptides, Pfizer, Seattle Genetics, Genentech/Roche, AstraZeneca, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties: CAR T-cells and biomarkers of cytokine-release syndrome; GlaxoSmithKline and Novartis: Research Funding. Kelly:Celgene: Honoraria; Genoptix: Consultancy; Spectrum: Consultancy; Pharmacyclics: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Epizyme: Honoraria; Karyopharm: Honoraria; GSK: Honoraria; BMS: Honoraria; Seattle Genetics: Honoraria; Gilead: Honoraria; Verastem: Consultancy; AstraZeneca: Consultancy; Sanofi-Aventis: Consultancy; Denovo Biopharm: Consultancy; Amgen: Consultancy; Takeda: Research Funding; Oncolytics Biotech Inc: Research Funding; Berkley Lights: Current equity holder in private company; Agios: Current equity holder in private company. Durie:Celgene/BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Orlowski:CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company; Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal